Background
There is limited prospective or comparative data evaluating the HRQoL impact of intensive chemotherapy (IC) in older adults (age ≥60 years) with AML. In the recent EA E2906 phase 3 study of '7&3' (daunorubicin & cytarabine) vs. clofarabine (CLO), a putative lower intensity therapy, we incorporated a prospective patient-reported outcomes (PRO) assessment of HRQoL and fatigue to understand treatment impact from the patients' (pts) perspective.
Methods
E2906 study design and results have been presented previously [n=727, med. age 68 years (range 60-86), randomized 1:1 to ‘standard’ ‘7&3‘ & high dose cytarabine (Arm A) vs. single agent CLO (Arm B), as remission induction (Step 1) and consolidation (Step 2), respectively]. There was no difference in composite complete remission (CCR, 50%) or 30-day mortality rates (8.5%), and in primary analysis CLO was inferior for Overall Survival. HRQoL was a key secondary protocol endpoint. PROs assessed HRQoL and leukemia-specific symptoms and concerns using Functional Assessment of Cancer Therapy-Leukemia scale (FACT-Leu), & Fatigue using FACT-Fatigue subscale. PROs were administered at prespecified time points: (1) Baseline; (2) Cycle 1 at nadir (approx. Day 15); (3) End of Step 1 restaging (approx. D 40); (4) Beginning of Step 2 (1 st consolidation, approx. D 45); and (5) 2 nd cycle consolidation/End Step 2 (approx. D 60). The FACT-Leu Trial Outcome Index ( TOI) was calculated by summing physical well-being, functional well-being, and leukemia-specific concerns subscales to create a single composite score. Higher TOI scores correspond to better HRQoL. The primary endpoint was to evaluate TOI difference from randomization to day 30 after induction therapy between arms, evaluated using Wilcoxon rank sum test. We also compared Clinically Meaningful differences (CMiD) in TOI (defined as patient change ≥½ standard deviation from baseline TOI) between arms, using the X2 test. Pts not completing at least 2 assessment forms (including baseline) were excluded. Testing of constructs of Physical Well-being (PWB), Functional, Leukemia & Fatigue scales demonstrated high Cronbach's a values (a >0.7) except for PWB (a=0.672) at a single time point (End of Step 2).
Results
Baseline TOI was available for n=489, and 364 pts (n=182 each Arm) were evaluable in this planned analysis. Evaluating CMiD during Cycle 1 induction, pts in Arm B were less likely to experience ‘Worse’ TOI score (32% vs. 40.7%, Arm A), more likely to maintain stable TOI scores (49.1% vs. 39%, Arm A), and had a similar proportion of ‘Better’ TOI scores (20.3%, A vs. 18.9%, B) although differences were not significant (p=0.1389). Conversely in cycle 1 consolidation, more patients in Arm A experienced Better TOI (45.1% vs. 29.2%, Arm B), fewer had stable TOI (45.1% vs. 58.3%, Arm B), and rates of ‘Worse’ TOI were similar (9.8%, A vs 12.5%, Arm B), again differences were not significant (p=0.2616). There was no difference in Fatigue scores between Arms (p=0.49).
We evaluated whether TOI and Fatigue scores could predict the ability to complete protocol therapy. In univariate analysis baseline TOI (p=0.0059) and Fatigue (p=0.0057) were associated with Step 1 completion, however on multivariate analysis (adjusting for age, sex, and Arm) were no longer significant [TOI, Odds Ratio (OR) 1.03 (0.98-1.08, p=0.27); Fatigue, OR 1.03 (0.96-1.11, p=0.42)]. There was a borderline association of TOI with Step 2 completion [TOI OR 1.10 (1.00-1.23, p=0.06)].
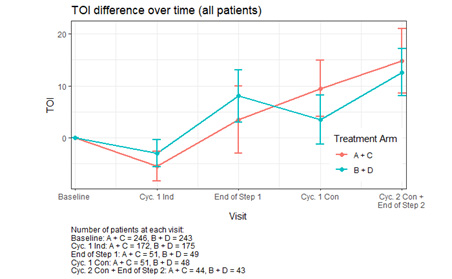
Using a multivariable linear mixed effects model, we observed similar and significant changes in TOI scores in both Arms, demonstrating the effect of IC & CLO on HRQoL (Figure). Comparing to baseline, TOI scores decreased significantly at C1 induction nadir (-4.0873, p<0.0001), and increased by 5.0762 (p=0.0004) at end Step 1, by 6.9047 at cycle 1 consolidation (p<0.0001), and by 11.4601 (p<0.0001) at end Step 2. Differences were mainly apparent in those who achieved CCR, and there was significant attrition in PRO responses in non-CCR pts.
Conclusions
Older adults experienced significant improvement in HRQoL after nadir regardless of therapy, and those achieving CCR report significantly higher scores. We did not observe differences in leukemia specific HRQoL or fatigue between treatment Arms (7&3 vs. CLO). Patient-reported HRQoL & Fatigue was not predictive of completion of protocol therapy. These results serve as a benchmark for expectations with IC in older adults.
OffLabel Disclosure:
Foran:Sellas: Research Funding; Roivant: Research Funding; Actinium: Research Funding; Novartis: Research Funding; Kura: Research Funding; Celgene: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Astellas: Research Funding; CTI: Membership on an entity's Board of Directors or advisory committees; NCI: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Luger:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria; Onconova: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Marker Therapeutics: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Novartis: Consultancy. Lazarus:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Altman:Syros: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kymera: Consultancy, Membership on an entity's Board of Directors or advisory committees; Curio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioSight: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kura Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; MD Education: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Research Funding; ALX Oncology: Consultancy, Research Funding; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Research Funding; Amphivena: Consultancy, Research Funding; Aprea AB: Consultancy, Research Funding; Aptose Biosciences: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Fujifilm: Consultancy, Research Funding; Kartos Therapeutics: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Telios: Consultancy, Research Funding; GlycoMimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cyclacel: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding. Pratz:Roche: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Jazz Pharamceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Research Funding; AbbVie: Consultancy, Research Funding. Tallman:Orsenix: Research Funding; Kura: Consultancy; Novartis: Consultancy; American Society of Hematology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Innate Pharmaceuticals: Consultancy; HOVON: Membership on an entity's Board of Directors or advisory committees; Ipsen Biopharmaceuticalas: Consultancy; Rafael Pharmaceuticals: Research Funding; Glycomimetics: Research Funding; BioSight: Research Funding; Amgen: Research Funding; AbbVie: Research Funding; Foghorn: Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties.
investigational use cofarabine (NCI phase 3 trial)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal